ProductAll categories
Faq
A molecular sieve is a material containing tiny pores of a precise (0.3-2 nm) and uniform size that is used as an adsorbent for gases and liquids. Molecules small enough to pass through are adsorbed, while larger molecules are not. It is different from a common filter in that it operates on a molecular level. Any materials that have relative uniform pore size of molecular level can be regard as “molecular sieve”. Examples include zeolites molecular sieve, carbon molecular sieve. Most molecular sieves in practice today are zeolites.
Zeolites are microporous, aluminosilicate. The term zeolite was originally invented in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heated material produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek, meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. (Wikipedia)
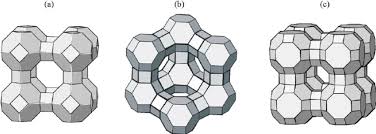
Zeolites are crystalline aluminosilicates of group IA and group IIA elements, such as sodium, potassium, magnesium and calcium. Chemically, they are represented by the empirical formula:
M2/n O.Al2O3.γSiO2. ώH2O
where y is 2 – 200, n is the cation valence and w represents the water contained in the voids of the zeolite. Structurally, zeolites are complex, crystalline inorganic polymers based on an infi nitely extending three - dimensional, four - connected framework of AlO 4 and SiO 4 tetrahedra linked to each other by the sharing of oxygen ions. As of September 2016, 232 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained has to be approved by the International Zeolite Association Structure Commission and receives a three letter designation.
